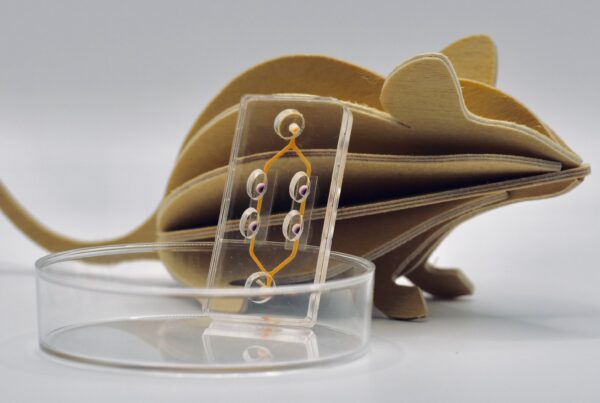
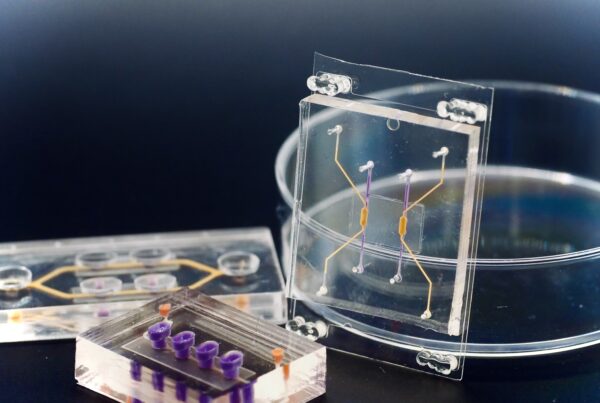
How do tumors respond to a specific therapy? Being able to answer this question before starting treatment would be incredibly valuable for cancer patients and their doctors alike. Researchers from the µOrganoLab, together with colleagues from the University Hospitals of Tübingen and the University Hospital of Würzburg, have made this possible — in real-time and using tissue from the patients themselves.

Precision Observation with Tumor-on-Chip Technology
Thanks to the innovative Tumor-on-Chip technology, doctors can now closely observe how tumor cells respond to therapy. “This allows us to individually assess how these tumor cells react to the planned treatment, what side effects may occur, and how these could be minimized,” explains Peter Loskill, head of the µOrganoLab and Professor for Organ-on-Chip Systems at the University of Tübingen and group leader at NMI Natural and Medical Sciences Institute in Reutlingen. This groundbreaking development has recently been published in the prestigious journal Cell Stem Cell.
The Tumor-on-Chip technology uses human cells to create a complex in vitro model of breast cancer. The team didn’t just replicate the tumor’s three-dimensional microenvironment; they also integrated a vascular-like perfusion system, simulating blood flow with an artificial blood substitute. Through this system, CAR-T cells were introduced to the tumor, and their effects could be directly observed.
What is CAR-T Cell Therapy?
Cancer cells are notorious for evading the immune system, which makes them so dangerous. Normally, T-cells, a type of white blood cell, are responsible for identifying and destroying foreign or harmful cells in the body. However, many tumors release signals that inhibit the activity of these T-cells.
CAR-T cell therapy tackles this challenge by isolating T-cells from the patient’s blood and then genetically modifying them in the lab to specifically recognize and attack the cancer cells. Once modified, these T-cells can not only target cancer cells effectively, but also remain in the body to combat the disease over the long term. Although the therapy holds immense potential, it does carry certain risks.
The Risk of Cytokine Storm
When CAR-T cells come into contact with cancer cells, they release cytokines — signaling molecules that recruit other immune cells to the tumor site. In some cases, however, this cytokine release can be excessive, leading to a condition known as Cytokine Release Syndrome (CRS) or a „cytokine storm„. This can trigger widespread inflammation, with symptoms like fever, chills, or nausea, and in severe cases, lead to organ failure or other life-threatening conditions.
Making Therapy Outcomes Predictable
This is where the Tumor-on-Chip technology proves its value. „The technology allows us to observe cells taken directly from the tumor we are targeting“ explains µOrganoLab PhD candidate Tengku-Ibrahim Maulana. „This means we can see how a patient’s tumor responds to CAR-T cell therapy and, additionally, how drugs might mitigate a cytokine storm, if it occurs.“
New Opportunities with Human-Based Model Systems
Organ-on-Chip technology, such as the Tumor-on-Chip model, enables the replication of complex human biological processes outside the body, while even capturing patient-specific variations. This breakthrough opens new doors, especially for novel therapeutic approaches like cell, antibody, and gene therapies. These technologies could allow for patient-specific assessments before clinical trials, offering cancer patients a completely new outlook on their treatment options.
Despite the promising advances, more research is needed to further develop this technology and bring it into clinical practice.
Read the full paper here.